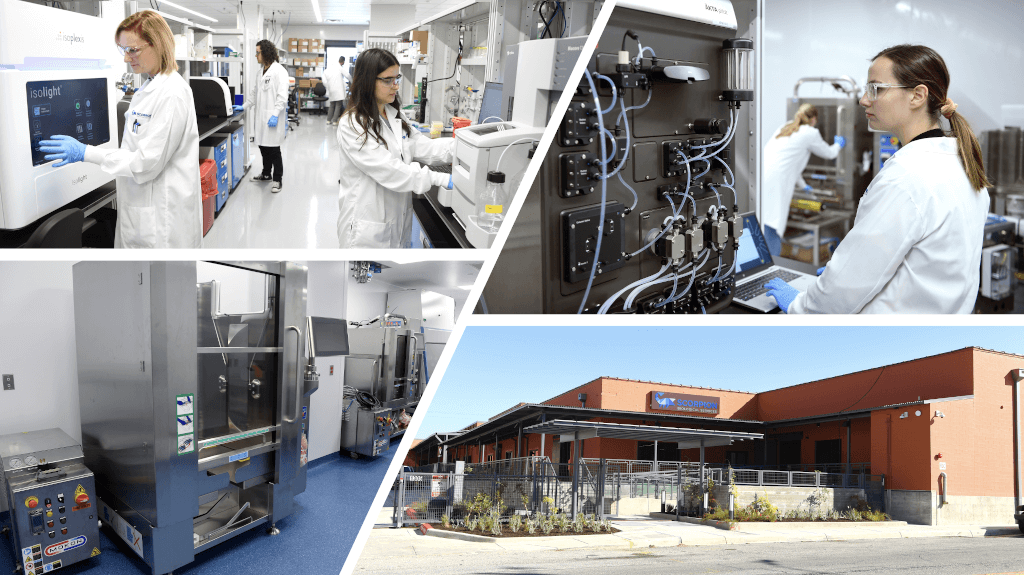
As an innovation-driven CDMO, Scorpius is committed to collaboration, efficiency and effectiveness. Our flexibly designed cGMP biomanufacturing facility embodies all three qualities.
We combine a modular approach to construction with the utilization of single-use systems to maximize our capabilities while minimizing the risk of cross-contamination in our multi-product facility. Scorpius delivers the support clients need across the full product lifecycle, from analytical method and process development through viral vector, mammalian and microbial manufacturing.